CABTREO CLEARANCE COULD BE AS CLOSE AS 12 WEEKS AWAY FOR YOUR PATIENTS1,2

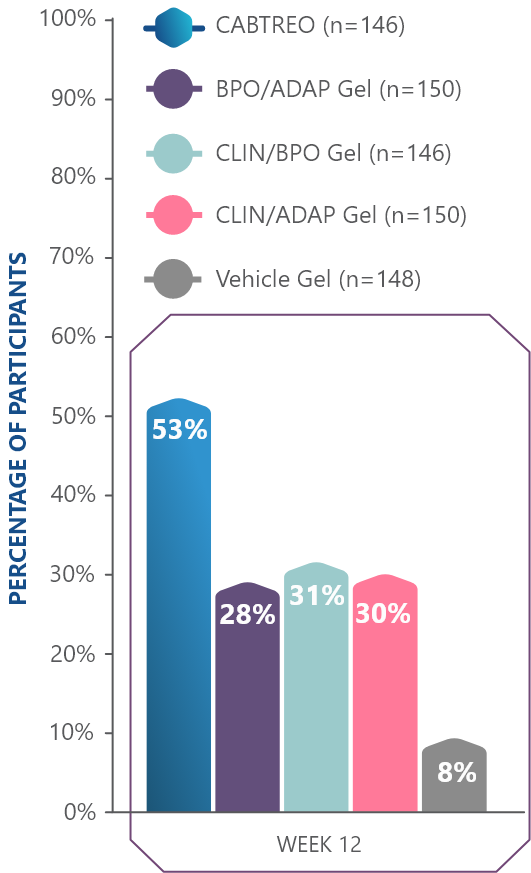
50% of patients had clear or almost clear skin at Week 12 in two Phase 3 clinical trials1,2
- In study 1, 50% of patients receiving CABTREO (n=122) achieved treatment success* vs 25% of those receiving vehicle (n=61)1,2
P<0.01
- In study 2, 51% of patients receiving CABTREO (n=120) achieved treatment success* vs 21% of those receiving vehicle (n=60)1,2
P<0.001
TREATMENT SUCCESS AS DEFINED BY EGSS THROUGH WEEK 12
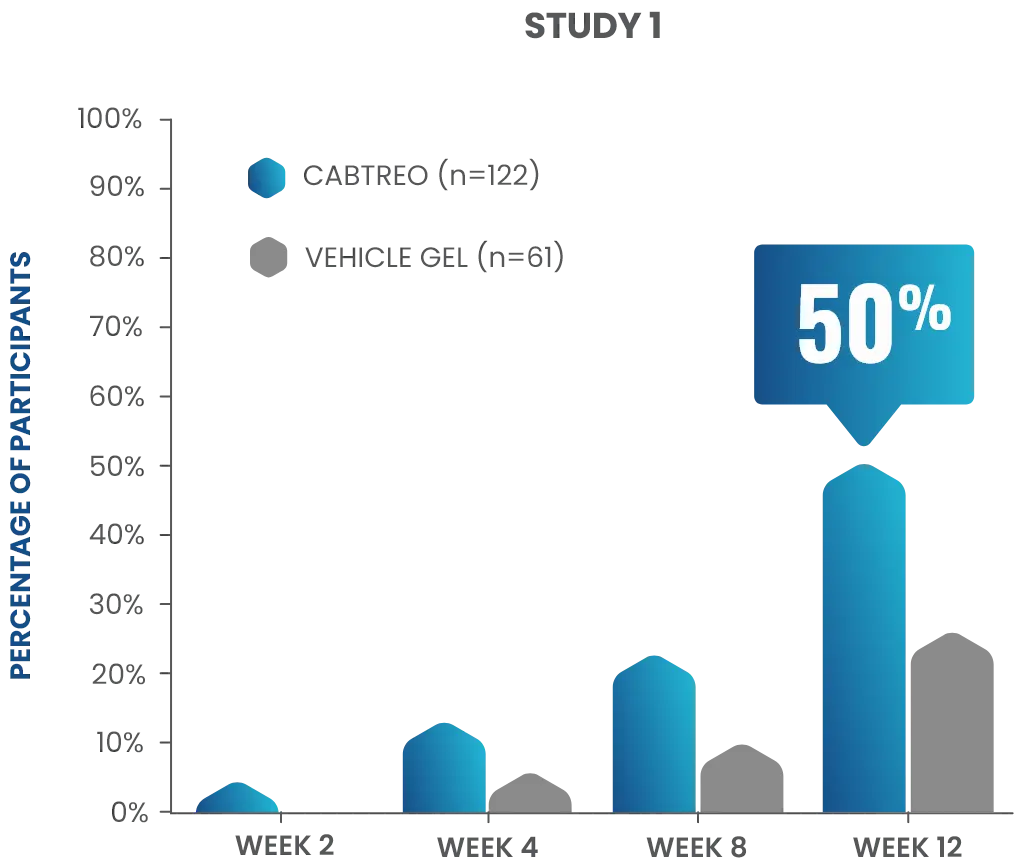
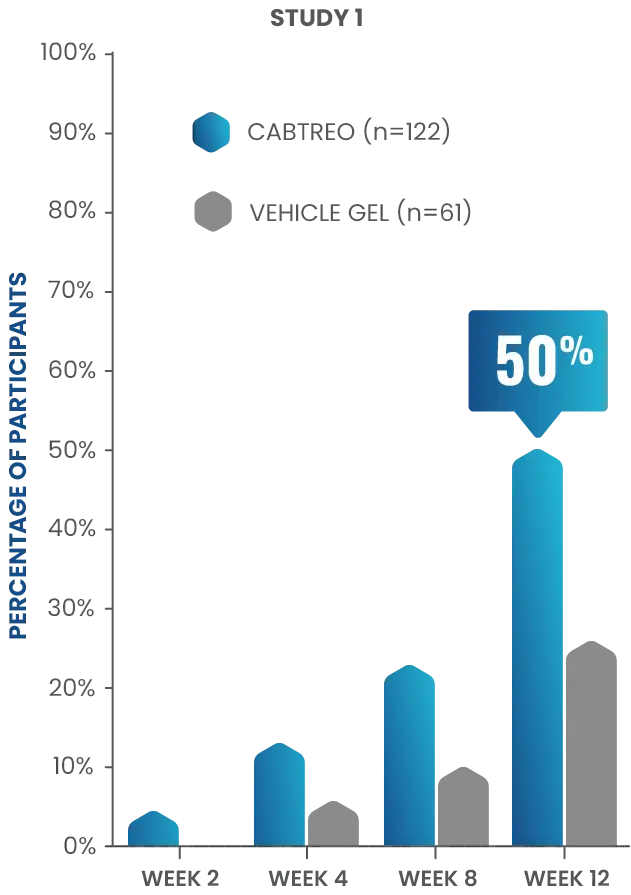
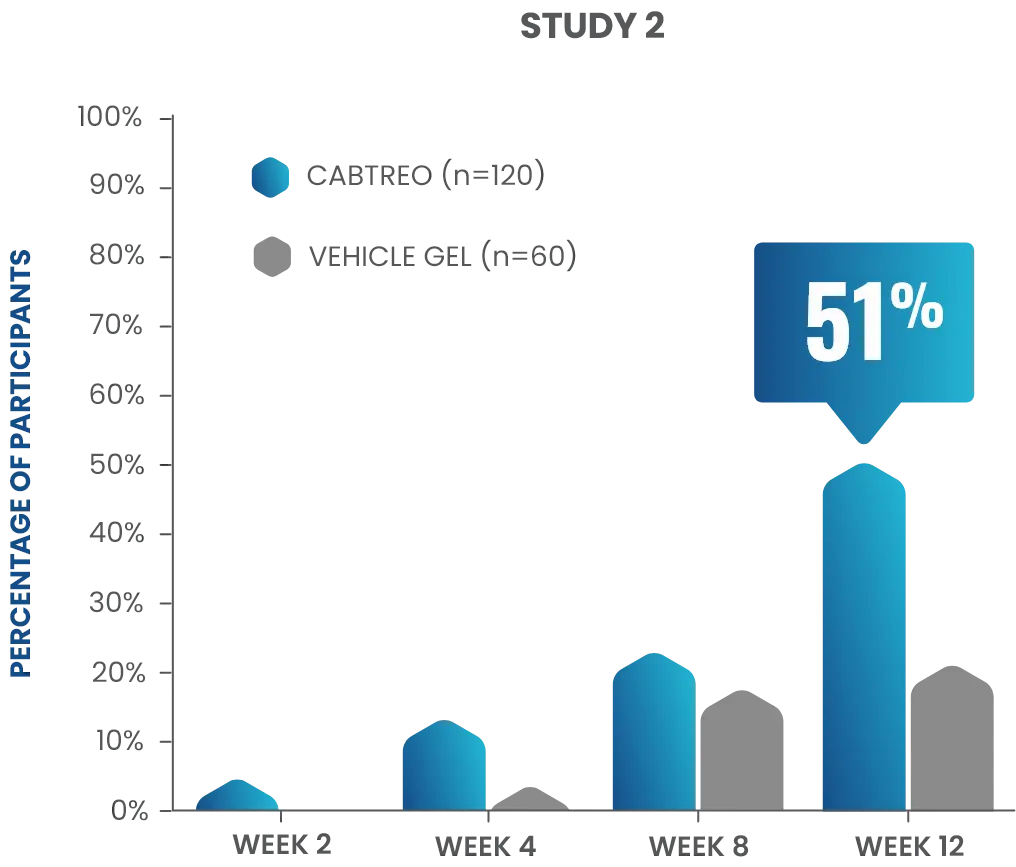
*Treatment success was defined as ≥2-grade reduction from baseline in EGSS and of a score of 0 (clear) or 1 (almost clear) through Week 12.1,2
EGSS, Evaluator’s Global Severity Score.
- The safety and efficacy of once-daily CABTREO were assessed in two multicenter, randomized, double-blind, vehicle-controlled, parallel group Phase 3 clinical trials of 363 subjects 10 years of age and older with facial acne vulgaris1,2
- Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 30 to 100 inflammatory lesions (papules, pustules, and nodules), 35 to 150 noninflammatory lesions (open and closed comedones) and two or fewer nodules1,2
- The coprimary efficacy endpoints of success on the EGSS, absolute change in noninflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 121,2
- While subjects aged 10 to less than 12 years were included in these trials, CABTREO is not approved for use in patients less than 12 years of age1
- The safety and efficacy of once-daily CABTREO were assessed in two multicenter, randomized, double-blind, vehicle-controlled, parallel group Phase 3 clinical trials of 363 subjects 10 years of age and older with facial acne vulgaris1,2
- Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 30 to 100 inflammatory lesions (papules, pustules, and nodules), 35 to 150 noninflammatory lesions (open and closed comedones) and two or fewer nodules1,2
- The coprimary efficacy endpoints of success on the EGSS, absolute change in noninflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 121,2
- While subjects aged 10 to less than 12 years were included in these trials, CABTREO is not approved for use in patients less than 12 years of age1


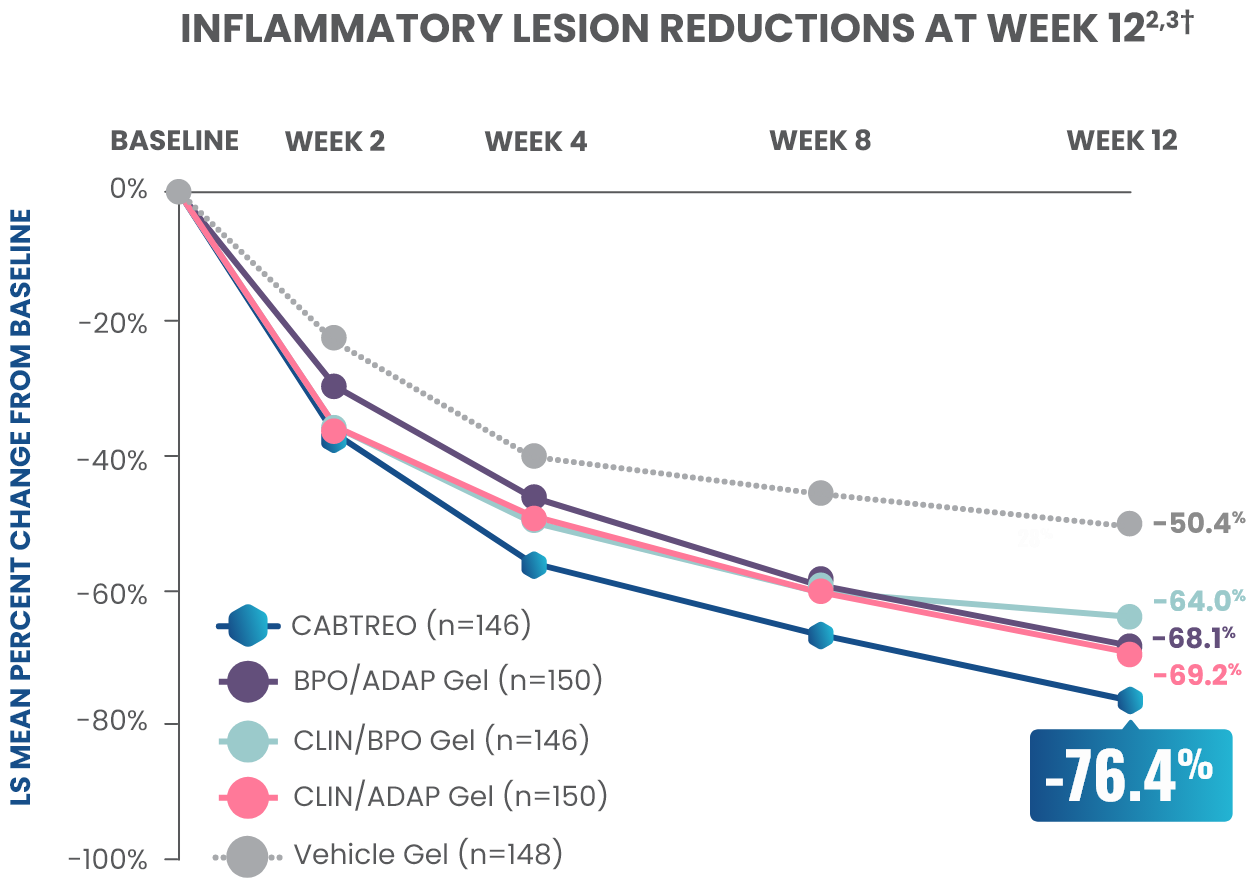
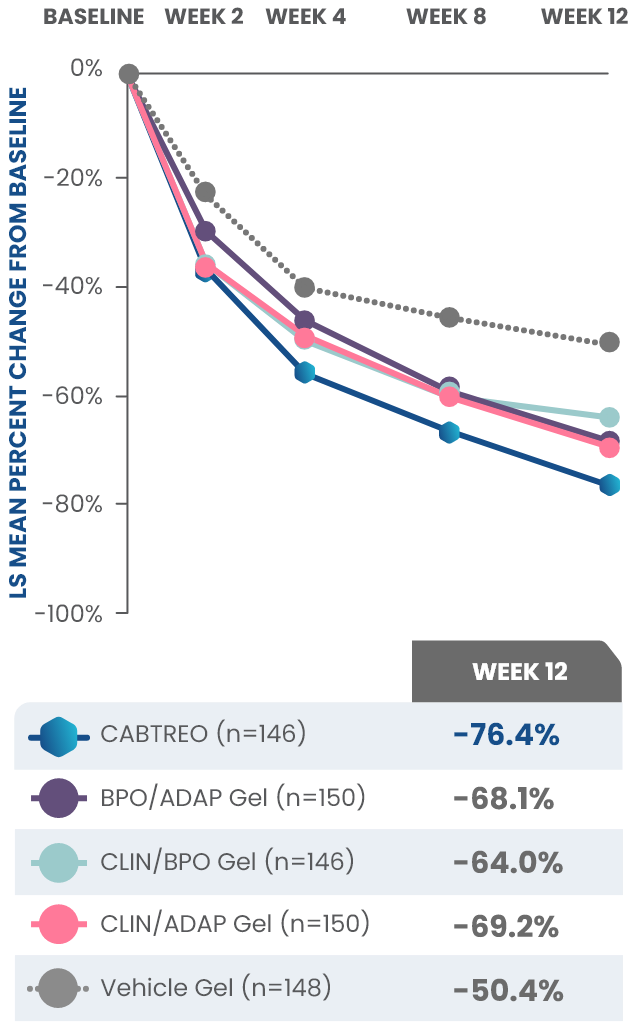
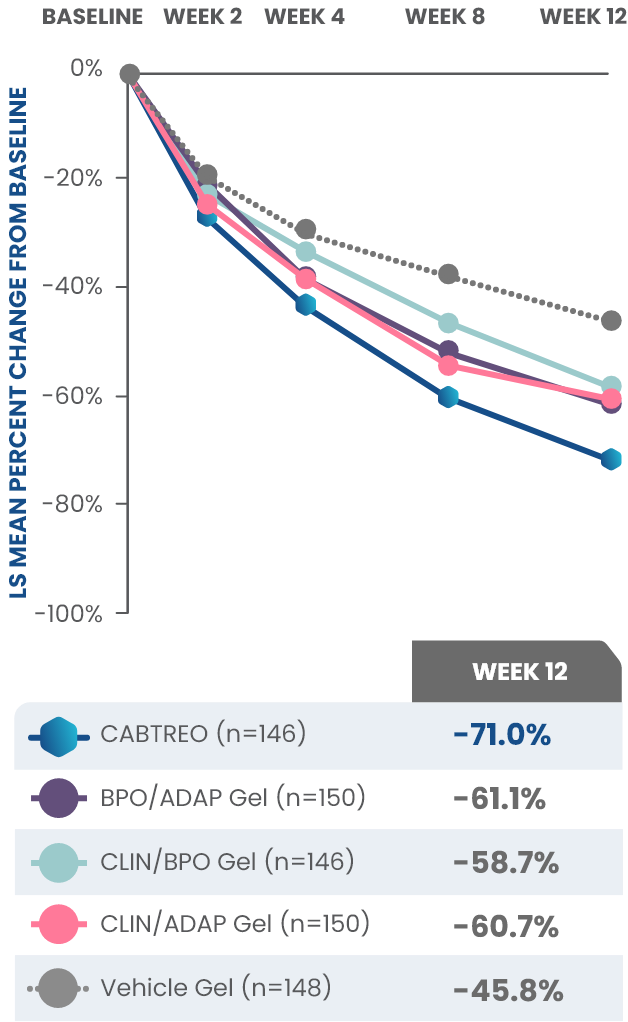
Statistically significant reductions observed across both trials
Up to 80% mean percent reduction in inflammatory lesions at Week 121,2
PRIMARY ENDPOINT: Mean absolute reduction in inflammatory lesions with CABTREO Gel was -28 vs -22 with vehicle in study 1 and -30 vs -21 with vehicle in study 2 (P≤0.001 in both trials)1,2
Mean Percent Reduction in Inflammatory Lesions At Week 121,2
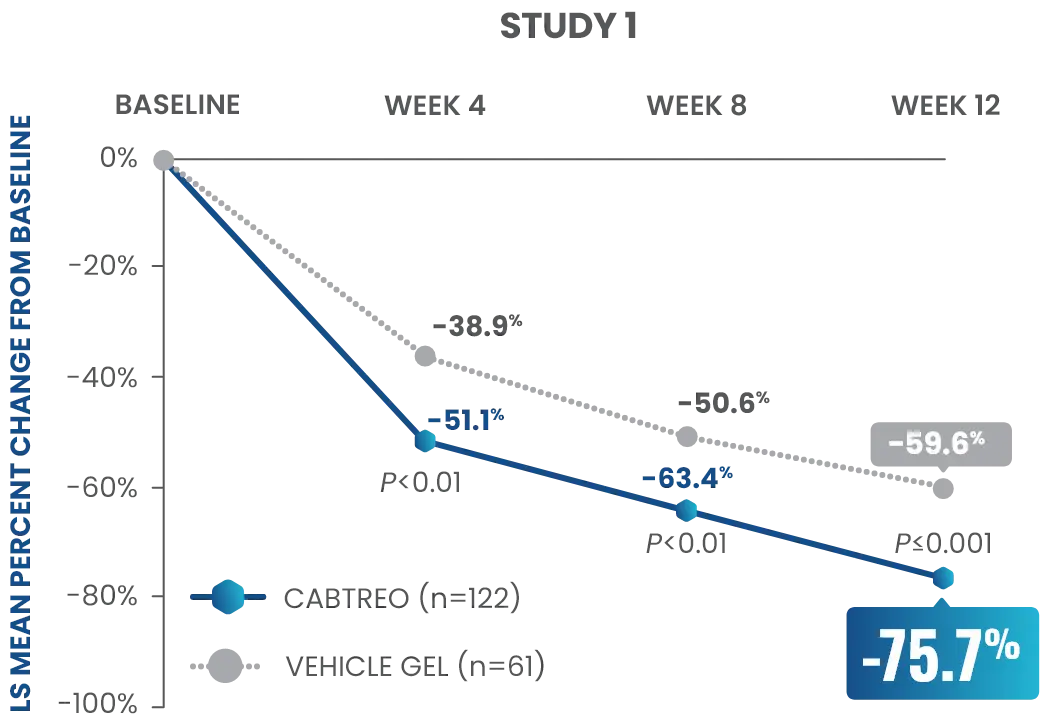
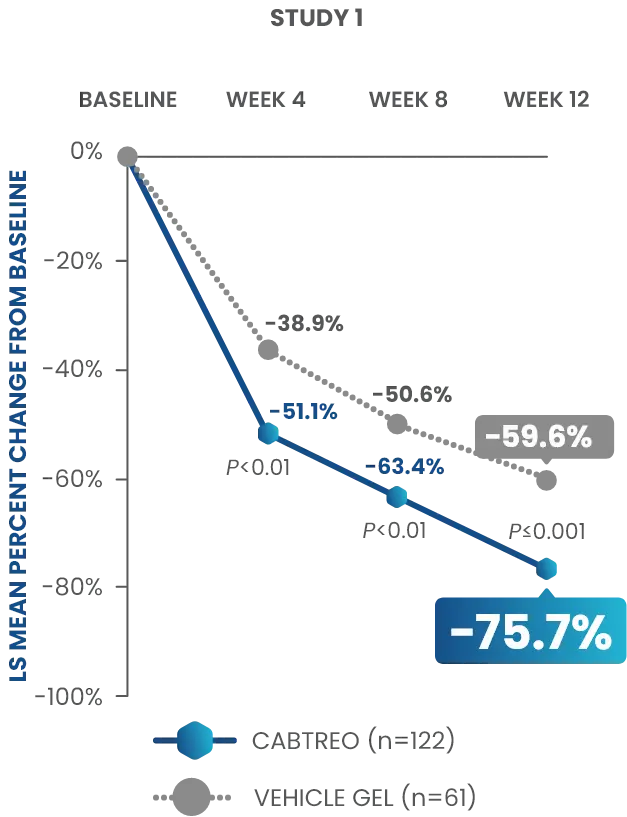

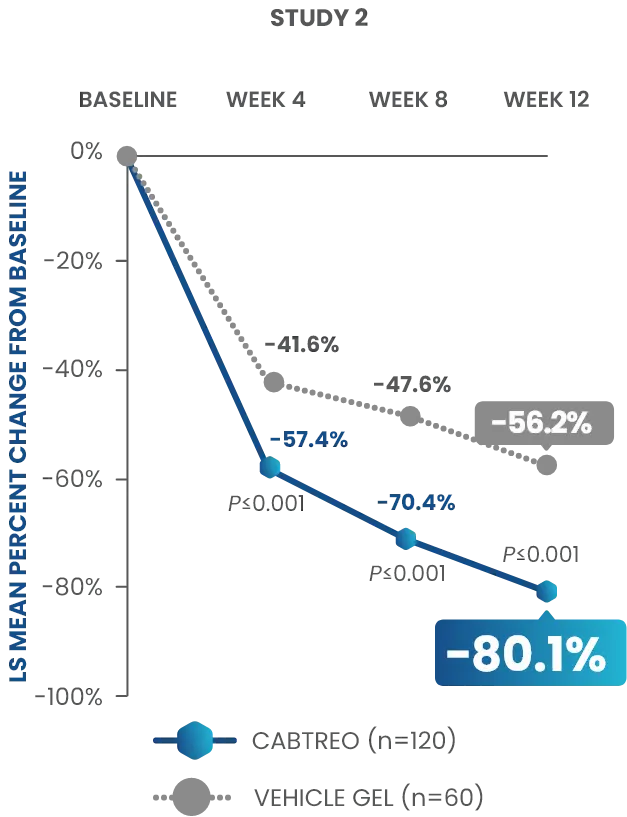
CABTREO, clindamycin phosphate 1.2%/benzoyl peroxide 3.1%/adapalene 0.15%; LS, least squares.
- The safety and efficacy of once-daily CABTREO were assessed in two multicenter, randomized, double-blind, vehicle-controlled, parallel group Phase 3 clinical trials of 363 subjects 10 years of age and older with facial acne vulgaris1,2
- Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 30 to 100 inflammatory lesions (papules, pustules, and nodules), 35 to 150 noninflammatory lesions (open and closed comedones) and two or fewer nodules1,2
- The coprimary efficacy endpoints of success on the EGSS, absolute change in noninflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 121,2
- While subjects aged 10 to less than 12 years were included in these trials, CABTREO is not approved for use in patients less than 12 years of age1
- The safety and efficacy of once-daily CABTREO were assessed in two multicenter, randomized, double-blind, vehicle-controlled, parallel group Phase 3 clinical trials of 363 subjects 10 years of age and older with facial acne vulgaris1,2
- Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 30 to 100 inflammatory lesions (papules, pustules, and nodules), 35 to 150 noninflammatory lesions (open and closed comedones) and two or fewer nodules1,2
- The coprimary efficacy endpoints of success on the EGSS, absolute change in noninflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 121,2
- While subjects aged 10 to less than 12 years were included in these trials, CABTREO is not approved for use in patients less than 12 years of age1
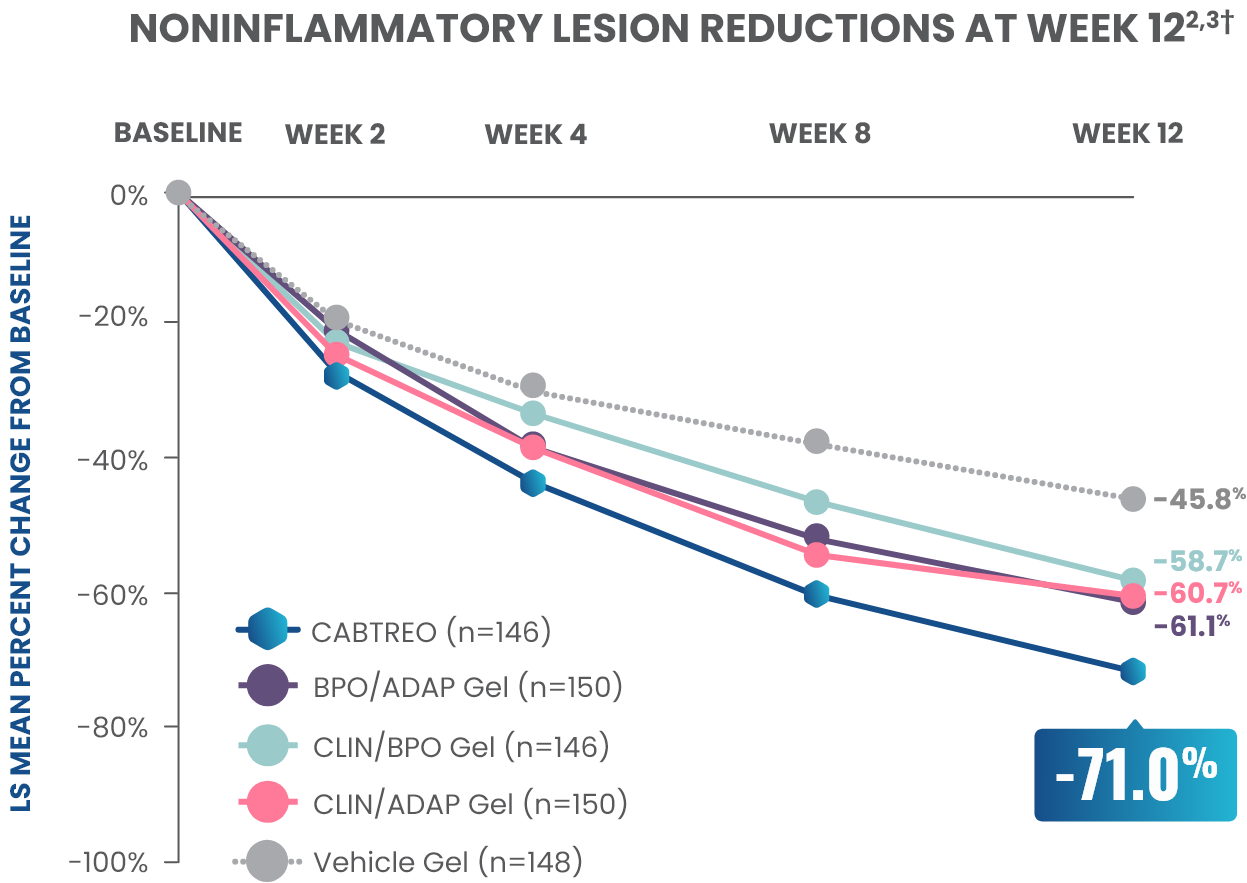
Up to 73% mean percent reduction in noninflammatory lesions at Week 121,2
PRIMARY ENDPOINT: Mean absolute reduction in noninflammatory lesions with CABTREO Gel was -35 vs -24 with vehicle in study 1 and -35 vs -22 in study 2 (P≤0.001 in both trials)1,2
Mean Percent Reduction in Noninflammatory Lesions At Week 121,2




CABTREO, clindamycin phosphate 1.2%/benzoyl peroxide 3.1%/adapalene 0.15%; LS, least squares.
- The safety and efficacy of once-daily CABTREO were assessed in two multicenter, randomized, double-blind, vehicle-controlled, parallel group Phase 3 clinical trials of 363 subjects 10 years of age and older with facial acne vulgaris1,2
- Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 30 to 100 inflammatory lesions (papules, pustules, and nodules), 35 to 150 noninflammatory lesions (open and closed comedones) and two or fewer nodules1,2
- The coprimary efficacy endpoints of success on the EGSS, absolute change in noninflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 121,2
- While subjects aged 10 to less than 12 years were included in these trials, CABTREO is not approved for use in patients less than 12 years of age1
- The safety and efficacy of once-daily CABTREO were assessed in two multicenter, randomized, double-blind, vehicle-controlled, parallel group Phase 3 clinical trials of 363 subjects 10 years of age and older with facial acne vulgaris1,2
- Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 30 to 100 inflammatory lesions (papules, pustules, and nodules), 35 to 150 noninflammatory lesions (open and closed comedones) and two or fewer nodules1,2
- The coprimary efficacy endpoints of success on the EGSS, absolute change in noninflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 121,2
- While subjects aged 10 to less than 12 years were included in these trials, CABTREO is not approved for use in patients less than 12 years of age1
Additional data:
See how CABTREO tested against dyad products in a Phase 2 trial

It’s time to move toCABTREO CLEARANCE
Additional data:
See how CABTREO tested against dyad products in a Phase 2 trial

It’s time to move to CABTREO CLEARANCE
Important Safety Information AND INDICATION
CONTRAINDICATIONS
CABTREO is contraindicated in patients with:
- known hypersensitivity to clindamycin, adapalene, benzoyl peroxide, any components of the formulation, or lincomycin.
- history of regional enteritis, ulcerative colitis, or antibiotic-associated colitis.
References: 1. CABTREO (clindamycin phosphate, adapalene and benzoyl peroxide) Topical Gel 1.2%/0.15%/3.1% [prescribing information]. Bridgewater, NJ. Bausch Health US, LLC. 2. Ortho Dermatologics. Data on file. 3. Stein Gold L, Baldwin H, Kircik LH, et al. Efficacy and safety of a fixed‑dose clindamycin phosphate 1.2%, benzoyl peroxide 3.1%, and adapalene 0.15% gel for moderate‑to‑severe acne: a randomized phase II study of the first triple‑combination drug. Am J Clin Dermatol. 2022;23(1):93-104.