Refrigerated distribution process prevents CABTREO from exposure to extreme temperatures1

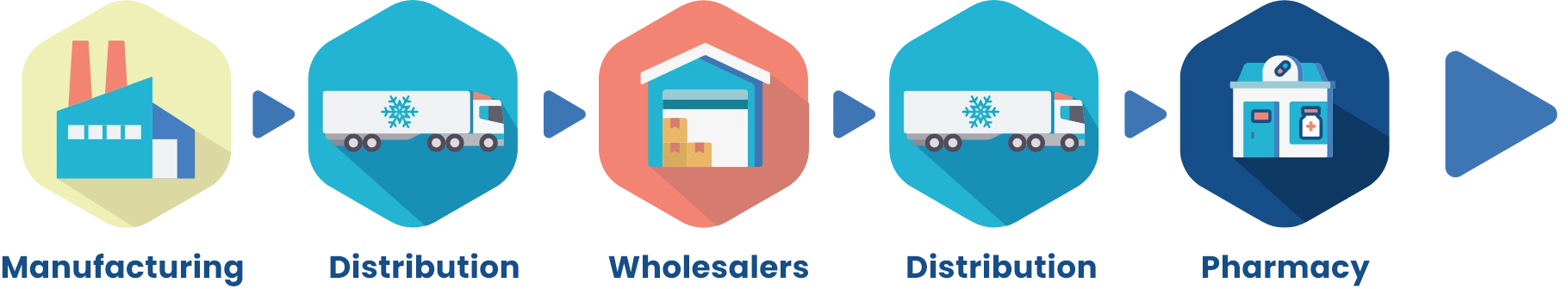
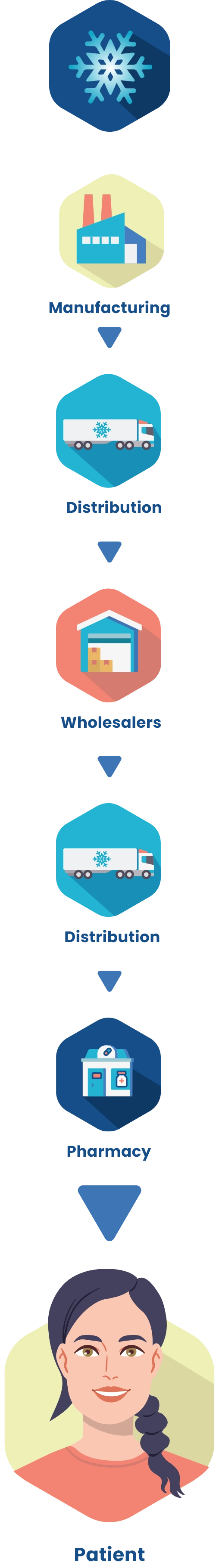
CABTREO is kept in cold chain (refrigerated) conditions between 2 °C and 8 °C (36 ° to 46 °F) from manufacturing until dispensed to the patient at the Pharmacy.
- After pharmacy dispensing: Store CABTREO at room temperature, at or below 25 °C (77 °F)
- Do not freeze
- Keep away from heat
- Store pump upright
- CABTREO is dispensed with a 10-week expiration date


ONE SCRIPT. ONE PUMP.
ONE APPLICATION A DAY.1
With one application a day, CABTREO can become a part of your patient’s daily routine.


Not an actual patient.
See More
Important Safety Information AND INDICATION
CONTRAINDICATIONS
CABTREO is contraindicated in patients with:
- known hypersensitivity to clindamycin, adapalene, benzoyl peroxide, any components of the formulation, or lincomycin.
- history of regional enteritis, ulcerative colitis, or antibiotic-associated colitis.
References: 1. CABTREO (clindamycin phosphate, adapalene and benzoyl peroxide) Topical Gel 1.2%/0.15%/3.1% [prescribing information]. Bridgewater, NJ. Bausch Health US, LLC. 2. Ortho Dermatologics. Data on file. 3. Stein Gold L, Baldwin H, Kircik LH, et al. Efficacy and safety of a fixed‑dose clindamycin phosphate 1.2%, benzoyl peroxide 3.1%, and adapalene 0.15% gel for moderate‑to‑severe acne: a randomized phase II study of the first triple‑combination drug. Am J Clin Dermatol. 2022;23(1):93-104.
